Try the MainEDC™ ePRO in decentralized and traditional clinical trials of all phases, pharmacovigilance source data capture, patient registries, marketing and feasibility studies. The system is accessible via mobile apps for iOS and Android or via a web-adapted page without installation.
Leverage your individual, flexibly configurable one-stop shop for simultaneous collection of clinical, drug safety and marketing data.
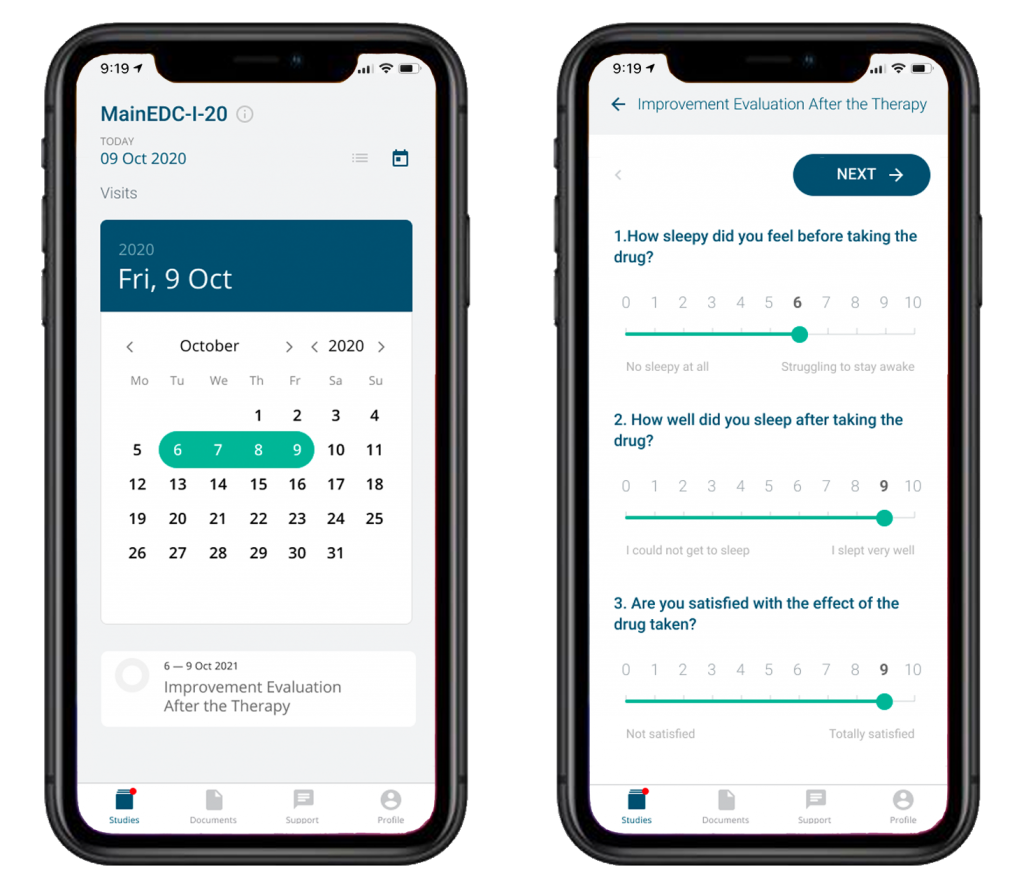
The system utilizes subject self-registration with or without the access approval step, which is not only time-saving but also allows to expand drastically the search for potentially eligible subjects, even bypassing clinical sites. Intuitive interfaces, good usability ensure users comfort and speed while entering data. MainEDC™ ePRO is fully integrated with the rest of the MainEDC™ platform but it can be used independently, too.
Apply your branding to the platform by labeling both applications at one go (mobile app and web-adapted page) with your logo and style. You can publish the app in the Apple Store and Google App Market under your company name.

Apply your branding to the platform by labeling both applications at one go (mobile app and web-adapted page) with your logo and style. You can publish the app in the Apple Store and Google App Market under your company name.
Instantly induce high customer loyalty to your brand name. Demonstrate to your clinical trial subjects and postmarketing research audience that you:
Invite your customers / study subjects to the web page or mobile app published under your company brand name.
The system supports electronic sign-off of informed consent, personal data processing and technical agreements.
Contact your customers directly, form your target audience, and use the collected data for new markets access. MainEDC™ ePRO will process up to 60 marketing indicators automatically:

MainEDC™ ePRO shares the platform with EDC, IWRS and Drug Supply components of MainEDC™, also supporting the MainEDC™ Hi Load technology for larger studies.
The platform has successfully passed benchmark testing and, what is even more valuable, its high scalability has been proven by real practical use in several clinical studies enrolling up to 70,000 subjects each.