MainEDC™ COVID-19 Pack
Data Management 365 urgently releases MainEDC™ COVID-19 Pack, a validated solution that supports clinical research in a pandemic environment.
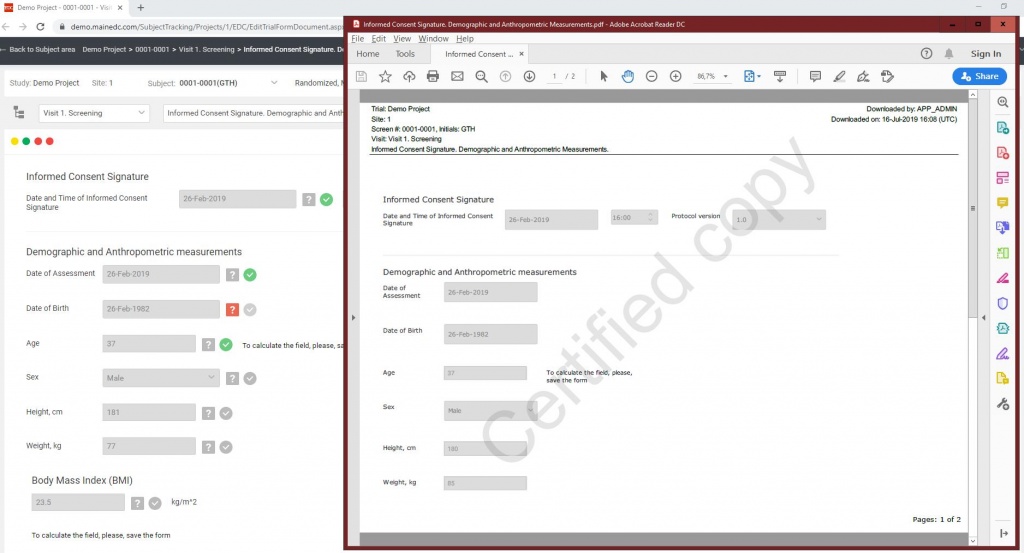
- Remote Monitoring with direct Source Data upload by Investigators ( Photo, Scan)
- Emergency changes due to possible deviations from original protocol
- Isolation of clinical sites
- Work from home
- Process continuity support
- Accelerated start of COVID-19 projects
For all our current customers, MainEDC™ COVID-19 Pack extension is free!
Our offer:
- Tools for implementing FDA* guidelines for clinical trials during COVID-19 Pandemic**.
- Full remote monitoring capability
- 24×7 from any device
- Instant access by the project team to the data entered by investigators
- Secure remote monitoring mode – Source Documentation Review Tool
- Project monitoring via recurring Email reports
- Paperless project support
- Validated electronic signatures
- QA support for research processes
- Keep your staff and Investigators informed at all times – Documents and Study News share tool (Email, SMS).
- Sponsors awareness (ALCOAC)
- Tools: Dashboard + KPI Report + Advanced Report Tool with 40 Predefined Reports
- Real-time data export to SDTM/SAS/XML/XLSX/CSV
- WHODrug, MedDRA, ATC, ICD coding and Auto-coding within the system
- Quickly start your COVID-19 study
- Extended support
- Full or partial project cloning
- Online eCRF approval in the system, without extra paperwork
- Instant implementation of protocol amendments
- Quickly update your study according to new requirements
- Dynamic design support
- Full control over Visit schedule
- Fast transition to EDC in projects running on paper CRF
- Protocol and Informed Consent Form versioning support
- Study paths support
- Hybrid study model maintenance
- Deviations control
- Protocol Deviations Tracker according to the New FDA Guidelines*
- Reshape monitoring
- RBM Dynamic settings
- SDV at any level:
- Field, Page, Visit, Subject, Site, Country
- Flexible rules for verification based on Site KPI, Confidence Interval, Country/Area Rules, Investigator Assessment
- Selection of Critical data for verification, review and approval
- Integration with 17 labs and 24 software vendors, full integration with Flex Databases CTMS
- We will help you reconfigure your system to suit new life scenarios
Our experience:
- Over 1,000 clinical trials on platform
- 32 studies with Central Monitoring
- 114 studies with Risk Based Approach
- 109 studies with partial SDV
- 218 studies without MV
- 100% of our clients use RBM-driven KPI Report
- 2 projects on COVID-19 already being configured with eCRF, randomization and logistics
We offer our validated solution:
- Compliance: GCP E6 R2, SAMP 5, 21 CFR Part 11, GDPR, HIPAA
- Validation: 36 QA, IT and Software Development Audits
- Security: 9 qualified Data Centers worldwide
- Advanced clustering, replication and backup procedures
- Audit trail based on blockchain technology
- 5 patents
MainEDC™ COVID-19 Pack will be included in the weekly release on March 25.
To rebuild your project or to start a new project, please contact us
* FDA Guidance on Conduct of Clinical Trials of Medical Products during COVID-19 Pandemic
** For more materials, please contact us Your Team Data Management 365
