News
Data Management 365 is proud to announce the launch of MainEDC™ AI Coder, an innovative AI-based tool designed to transform the medical coding processes in clinical research. Following the successful completion of pilot implementation and user evaluation, which demonstrated outstanding results in improving the efficiency and accuracy of coding raw textual data, MainEDC™ AI Coder […]

We visited IX Annual International Partnering-Forum “LIFE SCIENCES INVEST. PARTNERING ” on November 7-8, 2019.
DM 365 is happy to announce that now our unique tool developed based on our vast experience and professional trainings is available to all our clients.

Great to have warm traditions that get together all colleagues as a big friendly family. So, we follow Winter Health Days in our company every year.

We are happy and proud! On the first try Data Management 365 passed necessary and extended tests from our colleagues at Uppsala Monitoring Centre. We are now part of the WHODrug™ vendor program!

As we monitor and learn more about the COVID-19, we want to be transparent about what we’re doing as a company to protect our customers and employees.

Data Management 365 urgently releases MainEDC™ COVID-19 Pack, a validated solution that supports clinical research in a pandemic environment.
Emergency changes due to possible deviations from original protocol

Together we just broke the record for the quickest launch of a clinical trial. And now we can share the news!

Numerous clinical trials are being carried out on our integrated EDC / IWRS / Drug Supply / ePRO platform Today!
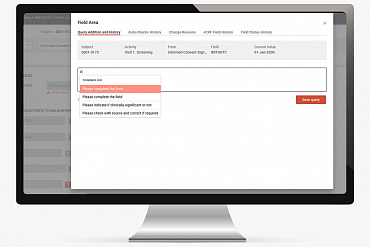
DM 365 team calculated the total number of manual queries that different investigators, CRA’s, data managers, medical monitors and coders created in MainEDC™. As a result, the new Query Templates feature was developed. The platform now offers 3 special tags and editable list of templates.
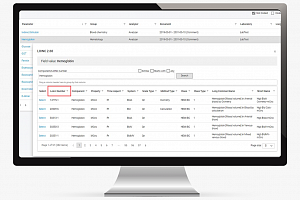
Data Management 365 journey of implementing LOINC in MainEDC™

Decentralized clinical trials, registries, marketing and feasibility projects with the new MainEDC™ ePRO – enjoy two interfaces (phone app and mobile-friendly webpage) working simultaneously with the integrated EDC / IWRS and Drug Supply system!

MainEDC™ supports Risk Based Monitoring processes in clinical trials

The most cited medical journal in the world, The Lancet, published an article featuring DM 365 as the provider of the electronic platform for one of the largest clinical trials ever conducted.

Data managers all over the world come across this question: how to handle the codes already assigned to eCRF terms when the next MedDRA / WHODrug version becomes available. Here is the answer

Data Management 365 is pleased to announce the evolutionary company transfer to Data Science standards.

Save up to 75% of clinical monitoring costs

We are excited to share our accumulated experience in the migration of data and eCRF structure in clinical trials

There is no need to say that every EDC business process could suffer from human error. Unfortunately, there is no way to prevent it, but there are ways to improve the process of data corrections.
In blinded trials, there are some situations where planned or unplanned unblinding is required. We decided to have a look at unblinding processes and share some thoughts from our experience.

MainEDC™ has been awarded a Summer 2022 Top Performer Award by SourceForge, the world’s largest software and services review and comparison website. This award recognizes exceptional companies and products with a significant amount of recent favorable user reviews that puts them in the top tenth percentile of highly reviewed products on SourceForge. To win the […]

In the end of the 2022 year Creative Pharma Company selected DM365 to be EDC vendor for their projects. We’ve asked Giorgos Tsakonas, Medical Data Manager a couple of questions about the upcoming partnership: What prompted the search for a new EDC vendor? We were looking for a new, cost-effective, simple but yet powerful EDC provider for our new projects. MainEDC fulfills all of our standards to be the “tool” we choose to use for our trials. What do you expect from working […]

From time to time, clients task the DM365 team with importing clinical data collected outside of MainEDC: from eSource, Excel spreadsheets, another EDC system that for various reasons the client decided not to use. As a result, we need to get a ready-to-use eCRF in the MainEDC system with automatically uploaded data.

Are you a sponsor forced to change the CRO on an ongoing project? We will do the data migration without stopping the study.